Date:Nov 2, 2020
In recent years, great success has been achieved in the development and application of metal-organic coordination supramolecular systems (mainly including metal-organic capsules (MOCs) and metal-organic frameworks (MOFs)). The research group of Prof. Duan Chunying from Zhang Dayu School of Chemistry in Dalian University of Technology (DUT) has been committed to the original basic research on the international frontier of inorganic chemistry and the national demand in the fine chemical industry. By constructing a metal-organic bionic structure containing amide chelate groups, the selective recognition and fluorescence response of small biological molecules such as carbohydrates are achieved, and a series of new high sensitive and sensitive materials are developed. Using the configuration of chiral metal center deployment and induced spontaneous resolution process, the combination of chiral hole space constraints, binding, and catalytic center development series high efficiency, high stereoselectivity catalytic and photocatalysis of new materials. Based on thestrong research foundation, Prof. Duan and Assistant Prof. Jin Yunhe from Zhang Dayu School of Chemistry have published their review article “Electron transfer in the confined environments of metal–organic coordination supramolecular systems” in Chemical Society Reviews (IF=42.8), a top international review journal of chemistry, which represents the leading position of DUT in relative research field.
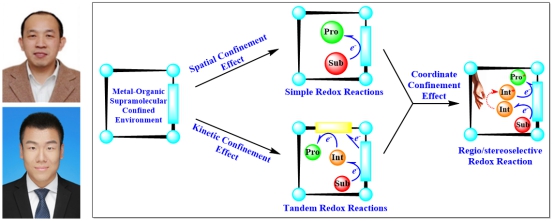
In this review,the group provides an overview of significant progress in the photophysical and catalytic applications of supramolecular metal–organic host–guest systems with electron-transfer processes in confined environments. Special emphasis is placed on the action modes and regulatory factors that affect electron transfer between different components to produce enhanced photophysical or redox catalytic performance. In their point of view, electron-transfer processes within the confined cavities are mainly controlled by spatial and kinetic effects. The spatial effect can accelerate confined-environment electron transfer via the close proximity of the components and control redox reaction regio- or stereoselectivity with the corresponding active sites. The kinetic effect can maintain active intermediates generated via electron transfer within confined active microenvironments and control the transfer of intermediates to nearby active sites for the subsequent reaction steps, including regio- or stereoselective steps, thus efficiently reducing the self-quenching of intermediates and byproduct generation. Both types of effects are important confined-environment electron transfer regulating factors and often act coordinately in catalytic systems. Finally, the prospects for confined-environment electron transfer, its application to photophysics and catalysis, and the remaining challenges in this field are highlighted.
Link to the paper: