Date:Dec 22, 2022
Recently, Niu Zanyao, an undergraduate from the cohort of 2022 majoring in Chemistry at Leicester International Institute, Dalian University of Technology (DLI), published his graduation thesis in the form of an academic paper on ACS Applied Materials&Interfaces, a famous journal in the material field of the American Chemical Society. The title of the paper is "Boosting Electrocatalytic Ammonia Synthesis of Bio-inspired Porous Mo-doped Hematite via Nitrogen Activation".
Niu Zanyao spent a whole year on researching of this paper and he completed all the experiment work, including data analysis, drawing, paper writing, and revision. The theoretical calculation was completed by Jiao Lei, the graduate of the School of Physics (Panjin). They are the co-authors of this paper. Niu Zanyao is currently studying for a master's degree at Imperial College London. This research was guided by Song Xuezhi, the professor from the School of Chemical Engineering,Panjin and Liu Lizhao, the professor from the School of Physics (Panjin).

Figure1. The structure of Mo-doped Fe2O3 and reduction
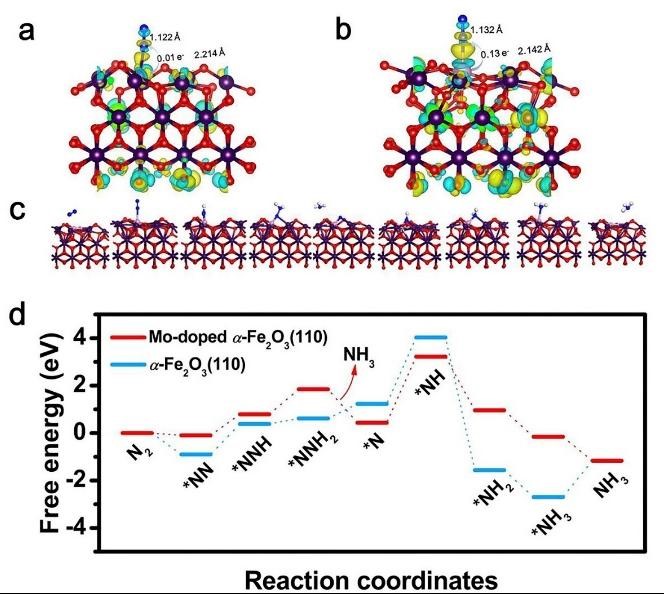
Figure2. DFT reaction
The abstract of the paper:
Electrochemical N2 reduction reaction (NRR) emerges as a highly attractive alternative to the Haber-Bosch process for producing ammonia (NH3) under ambient circumstances. Currently, this technology still faces tremendous challenges due to the low ammonia production rate and low Faradaic efficiency, urgently prompting researchers to explore highly efficient electrocatalysts. Inspired by the Fe–Mo cofactor in nitrogenase, we report Mo-doped hematite (Fe2O3) porous nanospheres containing Fe-O-Mo subunits for enhanced activity and selectivity in the electrochemical reduction from N2 to NH3. Mo-doping induces the morphology change from a solid sphere to a porous sphere and enriches lattice defects, creating more active sites. It also regulates the electronic structures of Fe2O3 to accelerate charge transfer and enhance the intrinsic activity. As a consequence, Mo-doped Fe2O3 achieves effective N2 fixation with a high ammonia production rate of 21.3 ± 1.1 μg h–1 mgcat.–1 as well as a prominent Faradaic efficiency (FE) of 11.2 ± 0.6%, superior to the undoped Fe2O3 and other iron oxide catalysts. Density functional theory (DFT) calculations further unravel that the Mo-doping in Fe2O3 (110) narrows the band gap, promotes the N2 activation on the Mo site with an elongated N≡N bond length of 1.132 Å in the end-on configuration, and optimizes an associative distal pathway with a decreased energy barrier. Our results may pave the way toward enhancing the electrocatalytic NRR performance of iron-based materials by atomic-scale heteroatom doping.